What’s a nice person like you, doing in a place like this? It’s not that bad, I think. In a totally unrelated matter, I attended a meeting with all of the administrative staff at Some Geek Told Me to discuss future ideas for blog posts.
The CEO came up with a few stupid ideas, the CFO mentioned a moronic suggestion, and the President of the company suggested a truly idiotic topic, though that’s pretty standard nowadays. However, whenever I need a dumb idea, the Vice President never disappoints me.
Because whatever the Vice President wants, the Vice President gets, so we are now going back to the Periodic Table. Yay! Our last venture into this elemental masterpiece was nearly four years ago, so if you missed it, check it out here before…I don’t have an answer to that statement.
Like me, I’m sure you’re a science fan, so how could you not love the Periodic Table? Created in 1869, by Russian chemist, Dmitri Mendeleev, the Periodic Table is the organisation of the known elements into a table, based on their physical properties, atomic number, atomic valence and their atomic mass.
One day I hope to understand it better, though at this rate, it might be the same time the All Blacks win the World Cup again.
So after reading this strange introduction, you would be forgiven if you’re wondering what is going on with today’s blog post. Other than neglecting my bed, we are going to start looking at where the names of the 118 elements have originated from, with the first part of this pointless exercise looking at real people.
Yes, 13 elements are directly named after real people, however, some different people are indirectly named after elements, but I will rant about them another day. I’m not going to present 13 elements in alphabetical order, as you would expect from this account, but rather in the order you would find them on the Periodic Table. So, without further fanfare, let’s turn the page and begin now.

Curium (Cm): Marie Curie and Pierre Curie
Curium is the 96th element on the Periodic Table, and it resides in the Actinoid group. It’s a synthetic element, which means it was created on purpose by Glenn T. Seaborg, Ralph A. James, and Albert Ghiorso in 1944, at the Lawrence Berkeley National Laboratory (Berkley), California, United States.
Curium was named after Marie and Pierre Curie, one of the most famous married couples in science. The Curies discovered polonium (Po), along with radium (Ra) and conducted years of research on radioactivity. In 1903, the Curies and Henri Becquerel shared the Nobel Prize in Physics, along with Marie winning the 1911 Nobel Prize in Chemistry.

Einsteinium (Es): Albert Einstein
Einsteinium is the 99th element on the Periodic Table, and it hangs out just three spaces along from curium in the Actinoid group. Einsteinium is another synthetic element, and it was created in 1952, by Albert Ghiorso, Torbjørn Sikkeland, Almon E. Larsh, and Robert M. Latimer, at the University of California, Berkeley, United States.
Using your amazing detective skills, you would have figured out that Einsteinium was named after, arguably, the famous scientist of the 20th century, Albert Einstein.
I’ve stated this before, but Einstein was a theoretical physicist who researched and published work on the photoelectric effect, Brownian motion and the Einstein relation, special relativity, the principle of mass-energy equivalence (E=mc2), statistical mechanics, general relativity, and many more outstanding contributions to science. Einstein also received a Nobel Prize in 1921 in Physics.

Fermium (Fm): Enrico Fermi
Since Fermium is the 100th element on the Periodic Table, it sits right next to Einsteinium in the Actinoid group. As you can guess, Fermium is a synthetic element, and in 1952, it was discovered by Albert Ghiorso and other scientists at the Lawrence Berkeley National Laboratory. It was created in the debris of the first hydrogen bomb explosion in 1952, code-named Ivy Mike.
Just like the previous two entries, Fermium was named after another 20th-century scientist, Enrico Fermi. He was a physicist, and among the various things he accomplished, Fermi helped construct the world’s first artificial nuclear reactor, as well as working on the Manhattan Project.
And just like the Curies and Einstein, he was also awarded a Nobel Prize, but this was in 1938 for Physics.
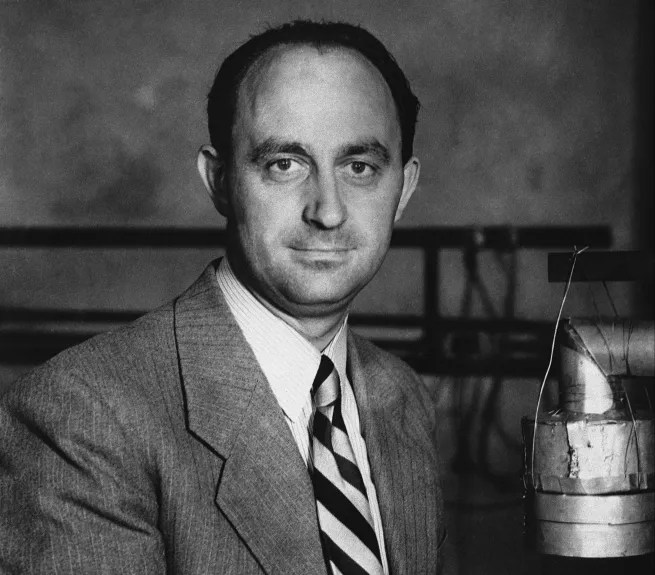
Mendelevium (Md): Dmitri Mendeleev
You knew this was coming, didn’t you? Mendelevium is another synthetic element and is the 101st element on the Periodic Table. It was discovered by Stanley G. Thompson, Albert Ghiorso, Glenn T. Seaborg, Gregory Robert Choppin, and Bernard G. Harvey in 1955 at the University of California.
Mendelevium was named after…wait for it…Dmitri Mendeleev! Mendeleev was the architect of the modern Periodic Table, and he did this by organising the elements into a table, based on their physical properties, like atomic number, atomic valence and atomic mass.
One remarkable aspect of Mendeleev’s Periodic Table is that he organised the elements in a way that allowed him to predict the existence of undiscovered elements by leaving gaps for them. History shows that his predictions were accurate with the later discoveries of germanium, gallium, and scandium.

Upload: Germansociety2014/Wikicommons
Nobelium (No): Alfred Nobel
As we march along the Actinoid group, we come across the 102nd element: Nobelium. Nobelium’s discovery seems to me, a bit odd. Scientists in Sweden announced the discovery of the element in 1957, but over the years, other sources have cited different years, such as 1958, 1963, and 1966, for the discovery. I don’t know who discovered nobelium first, because it may depend on who you talk to.
What isn’t up for debate is who nobelium was named after. Alfred Nobel was an inventor, engineer, businessman, and chemist who gave the world dynamite, gelignite, ballistite and the detonator, among other things. Nobel bequeathed his fortune to create a foundation to annually recognise people’s achievements for the benefit of humankind. Today, we know them as the Nobel Prizes.

Lawrencium (Lr): Ernest Lawrence
Lawrencium is the 103rd element of the Periodic Table, and like the others on this list, it’s another synthetic element. Similar to nobelium, lawrencium has been wrapped up in multiple claims of discovery. However, the earliest record of the discovery was in 1961, at Lawrence Berkeley National Laboratory, by Albert Ghiorso and a team of scientists.
Lawrencium was named after Ernest Lawrence, who was an accelerator physicist. He invented the cyclotron, which he was awarded the Nobel Prize in Physics in 1939, as well as worked on the Manhattan Project. He was also the founder of two laboratories, which were named in his honour after his death: the Lawrence Berkeley National Laboratory and the Lawrence Livermore National Laboratory.

Rutherfordium (Rf): Ernest Rutherford
As a New Zealander, this synthetic element has a special place in our rugby-obsessed hearts. Rutherfordium is the 104th element on the Periodic Table, and it’s a transition metal. Like the two previous entries, Rutherfordium has some controversy around its discovery. Whether it was discovered in 1964 by the Soviets or 1969 by the Americans, Rutherfordium only has a half-life of about 48 minutes.
Rutherfordium is the only element named after a New Zealander; my man, Ernest Rutherford. In 1908, Rutherford received the Nobel Prize in Chemistry for his research into the disintegration of elements and the chemistry of radioactive substances. He is also known as “the father of nuclear physics”, for his experiments that proved that the atom is made up of empty space, except for a small positively charged centre.
Rutherford proposed the term “nucleus” to describe the dense, positively charged core of an atom, thus giving him the title of “splitting the atom”, and the New Zealand $100 note was never the same.

Seaborgium (Sg): Glenn T. Seaborg
It may sound like an alloy from Star Trek, but I promise you it’s not. The 106th element on the Periodic Table is seaborgium, and it’s yet another synthetic element. If I have this correct, both Soviet and American scientists discovered seaborgium, independently from each other in 1974, so its another controversy surrounding the discovery.
As for the naming of the element, that is simple. Seaborgium was named after the chemist, Glenn T. Seaborg. Seaborg helped to discover 10 elements, plutonium, americium, curium, berkelium, californium, einsteinium, fermium, mendelevium, nobelium and the final one, element 106; which was eventually named seaborgium.
He also won various awards, including the Nobel Prize in Chemistry in 1951. Seaborg was responsible for adding the Actinoids strip on the Periodic Table. Cool.

Courtesy of the U.S. Atomic Energy Commission; photograph, Westcott
Bohrium (Bh): Niels Bohr
Sitting next to seaborgium in the transition metal group, we have the 107th element: Bohrium. There seems to be a trend happening because bohrium also has dual discoveries, with a Soviet team in 1976, as well as a German team in 1981.
People can argue about who discovered what element and when, but bohrium could have only been named after one person: Niels Bohr. Bohr was a Danish physicist, who also dabbled with philosophy. His contributions to science include atomic structure, nuclear fission, and quantum mechanics, but he also won the 1922 Nobel Prize in Physics.
The Niels Bohr Institute, at the University of Copenhagen is also named after him.

Meitnerium (Mt): Elise “Lise” Meitner
Meitnerium is the 109th element on the Periodic Table and another synthetic element. It was discovered by Gottfried Münzenber and Peter Armbruster at the GSI Heavy Ion Research Laboratory in Darmstadt, Germany in 1982.
There are only two elements on the Periodic Table named after real women, curium with Marie Curie, and the second is meitnerium, which was named after Lise Meitner. Meitner was a physicist who helped to discover the element protactinium, along with other discoveries like the Auger−Meitner effect and nuclear fission. She won the Max Planck Medal in 1949, the Enrico Fermi Award in 1966, and many other awards.

Roentgenium (Rg): Wilhelm Röntgen
The 111th element is roentgenium, and it’s another transition metal. Just like meitnerium, roentgenium was created at the GSI Helmholtz Centre for Heavy Ion Research Laboratory, by Peter Armbruster and Gottfried Münzenberg, but this time in 1994.
Roentgenium was named after the physicist Wilhelm Röntgen, sometimes spelt as Wilhelm Roentgen. Through his work, Röntgen detected a form of electromagnetic radiation with a wavelength shorter than those of ultraviolet rays and longer than those of gamma rays.
This radiation became known as X-ray or Röntgen radiation. This discovery meant that in 1901, he was awarded the very first Nobel Prize in Physics.

Copernicium (Cn): Nicolaus Copernicus
We are nearly at the end of the list, with only two to go! Copernicium is the 112th element and once again, was discovered at the GSI Helmholtz Centre for Heavy Ion Research. In 1996, a team led by Sigurd Hofmann and Victor Ninov worked for two weeks smashing lead with a beam of zinc ions, that were traveling at 30,000 km per second. The result of this experiment was the creation of a few atoms of copernicium.
If you’re a fan of space or astronomy, you may already know who I’m going to discuss. Copernicium was named after Nicolaus Copernicus, public enemy #1 for Flat Earthers. Copernicus was born in 1473 and achieved a lot in his life, which involved the fields of translation, medicine, economics, mathematics, laws, and diplomacy, but the main thing that he is still known for 500 years later, is his discoveries in astronomy.
Nicolaus Copernicus is often referred to as the father of modern astronomy due to his groundbreaking work, which demonstrated that not only is Earth a planet, but that other planets also orbit the Sun each year. In addition to this discovery, Copernicus explained that Earth rotates daily on its own axis. He also noted that very gradual changes in the direction of this axis are responsible for the precession of the equinoxes.
His model of the solar system is known as the Heliocentric system, which has the Sun at the centre, as opposed to the Geocentric model, which places the Earth at the centre.

Oganesson (Og): Yuri Oganessian
We have spent some time hanging out with the Actinoids and transition metals, but now we need to drift to the right, to visit the Noble Gases. Oganesson is the 118th and latest element to be added to the Periodic Table. It was created by a joint team of Russian and American scientists in 2002, at the Joint Institute for Nuclear Research in Russia.
Oganesson has three cool things going for it. It has the highest atomic number and highest atomic mass of all known elements, it was only formally named as late as 2016, and it’s the second element to be named after a living person, with the first being seaborgium and Glenn T. Seaborg.
And so, for the final time today, Oganesson was named after Yuri Oganessian, a nuclear physicist. His research on superheavy elements led him to help in the discovery of bohrium, meitnerium, hassium, darmstadtium, roentgenium, and copernicium. Oganesson has also been awarded the Lomonosov Gold Medal in 2017, and the Demidov Prize in 2019.

With the other 105 elements on the Periodic Table, their names will hopefully be explained upon multiple entries on this popular and well-respected website.
So that’s it for another blog post and another week. Thanks again for reading, following, and subscribing to Some Geek Told Me. Please remember to walk your dog, read a banned book, avoid breaking the spacetime continuum whenever possible, and I’ll see you next week.







































You must be logged in to post a comment.