I want to talk about the Periodic Table. Why? Why wouldn’t I, it’s the Periodic Table. You see, I’m a not a smart man. There are a lot of things that escape my attention and observation or things that fly over my head, in terms of comprehension. I struggle to understand Contemporary Art, the Floss dance, comic book reboots, Sour Cream and Chives, and the list could quite possibly go on for eternity.
However, with the Periodic Table; at least to me, it makes sense. Also it’s gorgeous. Just gorgeous. I think the reasons I love it are varied and confusing. From the arrangements, the groups, and…oh, I just love it. The point is, much like River Song’s speech in the “The Husbands of River Song”, when you love science, it’s like loving the stars themselves. You don’t expect the Periodic Table to admire you back. It’s a thing of utter beauty.
Now I will never confess to being an expert on the Periodic Table. That will never happen. Ever. In saying that, I do love the concept and the visual element1of the table, though. It makes sense once you understand it; though to be fair, while I was taking chemistry at high school, it drove me crazy looking at it. I also want to address something, that I will be using upper cases letters when writing the Periodic Table. This is because, in my tiny and humble opinion; it deserves it.
I also think it’s a status symbol. By this I mean, if you see the Periodic Table on someone’s wall; and they are not studying chemistry, that is bad arse. They could have pinned up a poster of their celebrity crush, favourite sports team or favourite film; but no. To me, anybody that has the Periodic Table or a map on their wall, screams “Yes, I am a geek, but I’m also a bad arse.”
It’s so important to the scientific community, that the Periodic Table has transferred over to pop culture, where you can find on mugs, t-shirts, socks and quite anything else you can think of. Yes, it has even crossed into the world of tattoos. For people with these tattoos, their badarsery is dialed to up to 11. Spinal Tap anyone?
The creation of the Periodic Table, to me at least; is one of the greatest intellectual and beautiful achievements in human history. Now for those of you that don’t know, the Periodic Table is also known as the Periodic Table of Elements. This table displays all of the known chemical elements (solids, liquids and gases) and organises them, for better understanding of their relationships to each other.

Before I go on, I want to talk about why the table exists in the first place. The short version is that for thousands of years, people knew about different materials that could behave in certain ways. Through experimentation, they could identify what the materials could do and what would be the best way to use that resource.
Over time, these materials were described as elements and they were studied by scientists for hundreds of years, to discover their properties, by identifying and recording them, collectively. 56 elements were known to be discovered, by 1863; and in 1869, the number had risen to 62.
Previously, scientists had tried to organise the known elements by their atomic weight2, but also by their atomic valence.3 Then along came Dmitri Ivanovich Mendeleev or simply, Dmitri Mendeleev.4 In 1869, this Russian chemist organised the elements into a table, based on their physical properties, atomic number, atomic valence and their atomic mass.5
However, as I have just stated in the above paragraph, other scientists had created similar tables before, so how was Mendeleev’s version any different? Why are we talking about his table and not someone else’s? It’s simple, because Mendeleev was operating on a whole other level: his table was full of predictions.
Now I’m not saying Mendeleev had a mutant power that allowed him to see the future; otherwise he would have joined the Winter Guard or Red Trinity teams or been a Soviet Super Soldier agent. No, but he did wield a power though, the power of science. What Mendeleev did was organise the known elements at the time, in such a way that he could predict elements that would fit into his table, that had not yet been discovered.
And do you know what’s funny about Mendeleev’s predictions? It’s not that he was wrong; it’s the fact that he was correct. If any future elements are discovered, they will easily fit into the Periodic Table, because of Mendeleev’s work. Oh, I need to add that this has already happened.
Bad. Arse. Seriously, if Mendeleev bumped into the Punisher or the Terminator in a narrow dark alley, the Punisher would step aside and the Terminator would beg for forgiveness.

Image by ExplorersInternational from Pixabay
Before we go any further, I just need to reinforce that I am not an expert on the Periodic Table. The following rant is how I understand the Periodic Table, so if I have got something wrong in my explanation, please feel free to explain it to me, in a polite manner on how I’ve messed up. Cool?
Anyway, let us now move our gaze onto the table itself. There are 118 elements on the table, with the first 94 elements being naturally occurring. As for the rest, elements 95-118 have been synthesized through different methods.6
The elements are sorted in the table according to their atomic number; which relates to the number of protons inside their nucleus. Examples are Hydrogen is 1: it has 1 proton; Selenium is 34: it has 34 protons.
When you look at the table, you can see the atomic numbers start to increase, as you move along a row, from left to right. Where the element is placed on the table, also relates to the column it’s in, because the element will react similar to other elements around it.
Easy enough, isn’t it? Alright let’s push on with what each tile or square means. Now each version of the Periodic Table can be slightly different, but they generally have the same things in the tiles. For an example, we have Boron. First you have the name of the element: Boron. Next you have the chemical symbol of the element, which is a shortened version of it’s name: B. The next part is the atomic number (the number of protons in the nucleus of the atom), by which the element is arranged on the table: 5.
Some Periodic Tables have an extra figure on the tile, which related to either the atomic mass, relative atomic mass or the mass number of the element. In this version, the figure is the relative atomic mass, which is the average mass of the element’s isotopes. So for Boron, it’s 10.806 or 10.81. Some tiles also have the element’s boiling and melting point in either Kelvin, Fahrenheit or Celsius.7
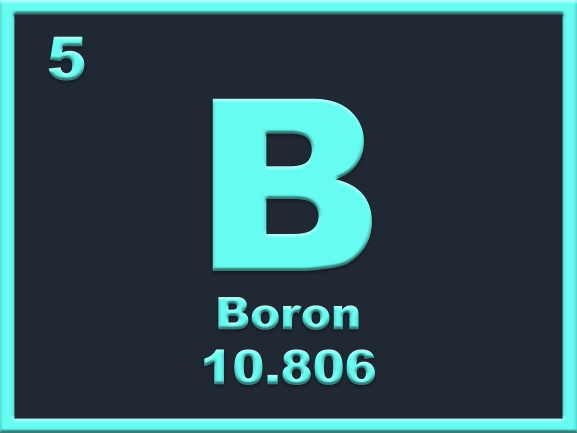
Image by Derrick Sherrill from Pixabay
Pushing on with this twisted love letter to the Periodic Table, the elements are divided into different sections. The horizonal rows are called periods and the elements in each row, all have the same number of electron shells or electron orbitals. The vertical columns are called groups. Within these columns, the elements here have the same electrons in their outer shells. This means they can react in ways that are very similar.
There are also four blocks that divide the elements up, which are s-block, f-block, d-block and p-block. They are grouped for their similar valence electrons orbitals.
Also on the right hand side of the table belongs to the non-metals, while the left hand side is for the metals. Clear as mud? Great, let’s look at how the elements relate to each other within the periods and groups.
Group 1: Hydrogen and Alkali Metals
1st Period
Starting at the top of Periodic Table is Hydrogen. If the Periodic Table was a fantasy/sci-fi novel or film, Hydrogen would be the Chosen One. Although Hydrogen has only 1 proton and 1 electron, it is also very different from the other elements, because it has zero neutrons. Not one.8 Because Hydrogen only has 1 electron in it’s outer shell, it is a very reactive and explosive gas. An obvious example of this is the Hindenburg disaster of 1937.
If the Periodic Table was a singles party, Hydrogen would be the hot mess that you would want to talk to and chat up; but equally being told by your friends to stay the hell away from them; it’s not worth the drama.
Underneath Hydrogen sits the Alkali Metals. These elements are very reactive, soft, shiny metals. All of these elements have 1 electron, so at the singles party, they would be ready to mingle and hook up. This makes them highly reactive.
Group 2: Alkaline Earth Metals
Next to the Alkali Metals, we have the Alkaline Earth Metals. These elements are white-silvery reactive metals, but less reactive that Alkali Metals. They have 2 electrons in their outer shells, so at the singles party, they would be the ones with full time jobs; they’re very stable.
Groups 3-12: Transition Metals
This is the largest group on the Periodic Table. These elements cover a wide range of metals, but a lot of them are very profitable; plus they have high boiling and melting points. Because of this, they can form alloys with ease; they are strong and hard; and are extremely effective at conducting heat and electricity.
At the singles party, they would be the bankers and models; people with a lot of money, that love throwing it around. Basically you don’t hook up with them, they hook up with you. There’s a big difference.
Groups 13-16 are mixed with three different groups. This is because of their very similar properties and the number of electrons shells.
Groups 13-16: Basic/Poor Metals
The eleven elements that make up this group, are much like the Transition Metals, but a little different. They have a lower melting and boiling point; they are soft; but they are still great conductors of electricity and heat.
At the singles party, these elements would be out to have a fun night, but they would not be flashing their money about, like the Transition Metals; they just don’t need to.
Groups 13-16: Metalloids
This group of elements has seven members and they all have something in common: they have metallic and non-metallic properties. Even though they look like metals, they are brittle, but they make excellent semiconductors.
At the singles party, the Metalloids would be the group of people that arrive late to the party, then have to try and figure out where should they go and stand; so they stay at the bar, hoping to attract someone’s attention.
Groups 14-16: Non Metals
The elements in this group are Carbon, Nitrogen, Oxygen, Phosphorus, Sulfur and Selenium. Just like the Metalloids, they are brittle when solid, but they are very poor conductors of heat and electricity. Instead, they can be a gas, a solid or a liquid at room temperature; and they can be found mostly in the Earth’s crust, the human body and the atmosphere.
At the singles party, these elements would have been through some messy break up, so they have very low expectations about the night; they’re just glad to be out of the house and talking to people.
Group 17: Halogens
The halogens are amazing at combining with metals to form different salts. As you move down this group, the melting and boiling points of the elements increases. This means having Fluorine as a gas at the top of the group, with Iodine being a solid at the bottom of the group.
At the singles party, these elements will talk to you very easily; but if you upset or offend them, they will break or hurt you. Talk to these elements with caution, because what they really want, is to hook up with Hydrogen or one of Hydrogen’s friends.
Groups 18: Noble gases
These group of elements are made of different gases that are unreactive. They are colourless, odourless and non-flammable, which means they are great at thermal conductivity. At the singles party, even though they are lots of people want to talk to them, they will say no to everybody. They have very high standards and nobody would be good enough for them. They are there to be seen, nothing more.
There are two small sub-groups that I need to talk about. They are called the Lanthanoids and Actinoids; and they are located in f-block, underneath the Transition Metals. The funny thing is that they actually fit into the Periodic Table in the 6th and 7th periods of the 3rd group. They were moved to allow for more space on the Periodic Table.9
Lanthanoids and Actinoids
The Lanthanoids are mostly non-radioactive, while the Actinoids are mostly radioactive. Together they also have magnetic, synthetic and other forms of elements. At the singles party, these elements would be ultra mysterious. They would ask a lot of questions about you, but never give any answers about themselves. We need to know more about them, before we could trust them.

Image by Cape Town Science Centre
To end, I want to draw attention to what some of the elements have been named after. There are 118 elements on the Periodic Table and 19 of them are named after 20 people; with 15 elements being named after scientists.
41 elements are named after or named for locations: 32 elements are named after places on Earth, while 9 elements are named after objects in our solar system.
There are even 4 elements named after a small town called Ytterby, Sweden; where 8 elements were discovered. Hell, there’s even an element named after the Norse God of Thunder aka the superhero with the hammer, Thor.
However I want to discuss a very special element. In 1955, a team of scientists managed to create 17 atoms of an unknown element. They realised that this unknown element filled the spot on the Periodic Table, being the element with an atomic number of 101.
Because of this, the unknown element was given the name Mendelevium and the chemical symbol of Md, after the Father of the Periodic Table, Dmitri Mendeleev. If you ever need a definition or example of badssery, that is it.
Thank you so much for reading and see you in a fortnight!
1Pun intended.
2Atomic weight relates to the weighted average of the naturally occurring isotopes.
3Atomic valence relates to the number of electrons an atom can give, take or share.
4Mendeleev has also been spelt Mendeleyev or Mendeleef.
5Atomic mass relates to the total number of protons and neutrons in the atom’s nucleus.
6I needed to point out that humans have created these elements and not dolphins or mice. 42 geek points to you if you get the reference.
7We generally don’t use Fahrenheit in New Zealand, so every time I see it, my brain just wants to shut down.
8Though to be fair, I am not talking about isotopes.
9Chemist Glenn Seaborg was responsible for adding the Actinoids strip at the bottom of the Periodic Table. The element Seaborgium (Sg) was named after him.

You must be logged in to post a comment.